Information for Participants of QRC-PVD Clinical Trials
What is a Clinical Trial?
Clinical trials are research programs involving people who volunteer to test a new ‘treatment’. Clinical trials aim to find out whether medical, surgical or behavioural interventions work, and if they are safe and effective in management or prevention of a disease.
The word intervention is a common term used for new or improved treatments, management programs, new detection or prevention methods.
To determine if the intervention treatment is effective, clinical trials compare two or more groups that receive different treatments.
Why do we need Clinical Trials?
QRC-PVD clinical trials help to improve treatment for people with Peripheral Vascular Disease (PVD). Trials are a reliable way for researchers to make sure interventions are an effective way to treat PVD. New treatments will only become available when they have been proven to be effective and safe in people with PVD.
The results from today's clinical trials will help people in the future. Treatments you receive today are outcomes from previous clinical trials.
Further information about clinical trials is available on the Australian Clinical Trials website.
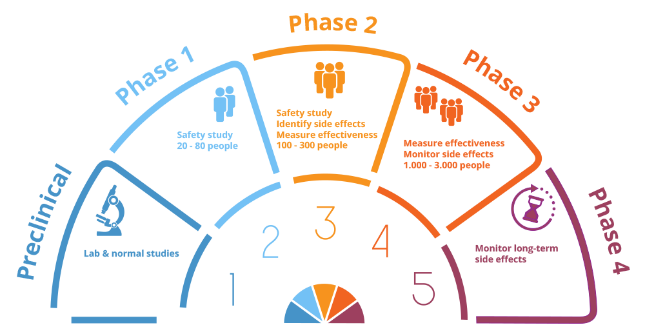
The phases of clinical trials.
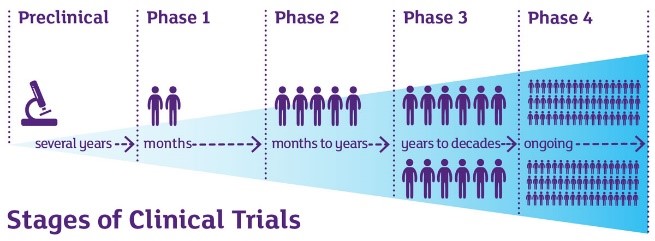
The stages of clinical trials.
Clinical trials conducted by QRC-PVD
The best way to test a new treatment for its effectiveness is through randomised control trials. In this trial, participants randomly receive the actual treatment or the control treatment (often called a placebo).
In a randomised control study, the researchers do not know who is receiving the actual treatment or the control treatment. This ensures the results of the trial are as accurate as possible and the two groups (new treatment and control group) are as similar as possible. The randomisation is carried out by an independent member of the research team (usually the study pharmacist).
When the researchers and the patient do not know what treatment they are receiving, it is called a 'double blind trial'. Blind trials makes sure the result of the study is not affected by this knowledge and increase the accuracy of the results.
In drug clinical trials, a placebo is a dummy treatment designed to look like the real treatment being tested. Placebos contain inactive ingredients, such as sugar, and the outcomes of patients on the real treatment is compared to patients on the placebo at the end of the study.
How a Clinical Trial works
A protocol is a plan or set of rules that clinical trials must follow to ensure participants are as safe as possible and that the correct measurements are taken to provide meaningful results.
The protocol contains information about the evidence to support the trial and provides details on:
- Who is eligible to take part in the trial
- What research methods, tests and procedures will be used
- What interventions will be used
- The length of the study, and
- The information collected and any follow up required.
The eligibility criteria describes the type of participant who is being sought. The eligibility criteria ensure participants are similar and the results are clear on who they would benefit. The criteria are there to keep participants safe and participants outside of these criteria are excluded as the risk may be too great.
Before participants are randomised into a trial, they will undergo a screening phase. This involves obtaining information about you that will:
- Inform the researcher if you are eligible
- Provide a baseline so that we have something to compare with, and
- Describes your characteristics.
The information collected during screening can be used to determine the level of change once the study is completed.
All participants in a clinical trial must consent to be part of the trial. Participants are given information on treatments, tests, benefits and risks before consenting to take part in the trial.
As a participant, you should ask any questions you have to make sure you completely understand what is involved in the trial. There is no such thing as a 'dumb' question and you are encouraged to keep asking questions until you are completely satisfied that you have all the information you need to make a decision.
You are also encouraged to discuss the trial with your GP, a friend and/or your family before you make your decision.
There are a number of people involved in clinical trials, including doctors, research workers and other health care professionals who will monitor your health carefully.
As a part of the trial you may have more tests and medical exams then you normally would. You may be asked to do other tasks during the trial such as, filling out questionnaires, forms or asked to keep a diary of your health.
You may also be required to travel to the hospital or university to undertake assessment in relation to the trial.
Taking part in a clinical trial can provide benefits such as access to new treatments, a higher standard of care and your health is closely monitored. Another benefit is that the trial you take part in will add to scientific knowledge which may become vital to improving medical care in the future.
Volunteering for a clinical trial will help others with similar health issues to yours. You will also be helping researchers from QRC-PVD answer questions which will help improve the quality of life of those with PVD.
Participation in clinical trials comes with some risks and downsides, such as:
- New interventions may not always be better than care already available
- The new intervention may not work for you, but does for others
- Unexpected side effects
- Some tests maybe uncomfortable, e.g. having a blood test.
Protecting Participants
The health and safety of participants is the highest priority for our researchers. Each trial has scientific, medical and ethical oversight from external reviewers to protect the rights of participants.
Australian clinical trials are regulated by law and codes of conduct which protects participants' rights and the integrity of the research.
Clinical trials in Australia are approved by a Human Research Ethics Committee (HREC) who check to ensure the trial confirms with the requirements of the National Statement of Ethical Conduct in Human Research.
Informed consent is a process where patients are provided with details of the trial, the protocol, the student information booklet and given an opportunity to ask questions or seek opinions from others.
Taking part in a clinical trial is your decision. By signing the informed consent document, you are agreeing to be a part of the trial however, you have the right to withdraw at any time.
Our health care professionals monitor your health throughout the trial and if they feel it is in your best interest to withdraw from the study, they will discuss this with you.
Funding
QRC-PVD seeks funding from various institutions to fund its research. Most of our funding is sought from government institutions including the National Health and Medical Research Council (NHMRC), health services such as Queensland Heath or charities such as Diabetes Australia.
We are also funded by private companies who ask the centre to collect data on the effectiveness of their interventions.
Completed Trials
Once a trial is completed, all data will be analysed and reviewed by the research team. Results of the trial will be published in journals to share information with other health care professionals.
Researchers may report progress of trials and its findings at scientific meetings, medical journals and authorising bodies (HRC). The institution funding the trial will also require reports of the outcomes of the trial.
The results status of studies will also be available on this website. It is not possible to provide or identify individual results, as the data collected is grouped according to allocation and analysed comparing groups. These grouped results will be used in publications and presentations.