Current & Recent Projects
The Trust has provided research grants to the following projects in recent years:
2023
Lead researcher: Nicola Thomson (Masters by Research student)
The Australian Box Jellyfish, Chironex fleckeri, is a significant threat to human health and the tourism industry in Australia. This deadly creature leads to the closure of popular beaches and bays for extended periods, impacting local tourism economies. However, the current methods of detection are often unreliable and time-consuming, relying on observation of the animals. To address this issue, researchers are turning to Environmental DNA (eDNA) as a revolutionary detection tool. eDNA involves the forensic detection of tiny amounts of DNA released by the target organism into the environment. It is cost-effective, rapid, and requires fewer resources than traditional methods.
eDNA allows the detection of organisms without direct observation and has already successfully identified several cubozoan species and their ecological characteristics. Now, efforts are underway to develop a rapid in-field eDNA sequencing technologies, with lateral flow assays (LFAs). This technique is showing promise for rapid and reliable detection of box jellyfish.
The specific aims of my project include the following: (1) developing and testing LFAs for in-field detection of C. fleckeri; (2) comparing the results with traditional lab-based workflows, and validating the in-field detection method at jellyfish hotspots. The research group has already developed a highly sensitive eDNA detection TaqMan assay for C. fleckeri, facilitating the development of the in-field workflow and related detection.

2022
Lead researcher: Professor Mike Kingsford/ Reef and Ocean Ecology Laboratory
An understanding of population structures is fundamental to understanding threats, where do jellyfish come from and how far do they go? If there is great genetic exchange among local populations then the source of jellyfish may be distant to areas where swimmers are threatened. In contrast, if populations are geographically small and, are not connected to other populations then the ability to make predictions on likely threats would be more robust. Our focus is on the deadly stinger, Chironex fleckeri, the species that poses the greatest threat to humans in tropical waters of Australia.
Population connectivity is the exchange of individuals among geographically separated population units. Some local populations may be limited to a small geographic area such as a bay, estuary or island. It is also possible that local populations with little or zero connectivity could contribute to a mosaic of genetic diversity within a stock, and under the right circumstances could become a separate stock (Pineda et al 2007).
Jellyfish have historically been classified as plankton. Accordingly, it would be assumed that connectivity would be high among local jellyfish populations and that populations would vary little in both ecological and genetic clade-level differences over broad spatial scales (i.e., hundreds to thousands of km). However, findings on the behavior and swim speeds of jellyfish suggest they may have swimming behaviour that more aligns with nekton (McManus et al. 2012). Nekton can swim to either maximize dispersal or minimize it by remaining close to natal areas and these strategies can spatially broaden or restrict species population structures, respectively.
Cubozoans have a number of attributes that assist in restricting the dispersal of medusa from localized areas. Although cubozoans are generally small, they are generally highly mobile. The fifty known species of cubozoans range in size from those with a bell diameter of a few cm to about 20–25 cm and all of the species that have been observed swim well. Chironex fleckeri (size range: 4 to 12 cm IPD) have been measured to swim at speeds of up to 16.6 cm s−1 in the field (Schlaefer et al. 2018).
Cubozoans have excellent sensory abilities including vision provided by 24 eyes. More complex behaviours of cubozoans include responding to objects, conspecifics, currents, diel cycles, shadow and light, bioluminescent plankton, habitat type and small-scale geography (Kingsford et al 2021).. Accordingly, predictions for population structures of jellyfish based on passive dispersal are likely to be highly inaccurate.
Our objective it to use mitochondrial DNA (mtDNA) to identify intraspecific phylogenetic relationships among local populations along the coast of Queensland. We will use this approach to study the gene flow and connectivity of local populations separated by 10s to 100s of kilometres and this will include multiple locations used by swimmers. Using this approach we will determine scales of connectivity.
Lead researcher: Emily O'Hara (PhD Candidate)
This study focuses on a species of box jellyfish, Carukia barnesi or the Irukandji jellyfish. Surprisingly little is known about this small jellyfish despite being first described in the 1960s and considering this species is responsible for the majority of hospitalisations from cubozoan stings in northern Australia. The clinical manifestations of C. barnesi stings cover a wide spectrum from mild, localised pain to near morbidity and a plausible reason for this diversity of symptoms is the intra-specific differences in the venom composition of the tentacle and bell nematocysts .
The main aim of this study is to examine the venom ecology of the juvenile stages (polyps and recently metamorphosed medusa) of C. barnesi, an animal for which strong circumstantial evidence suggests venom variation may occur over its development. I aim to achieve this by collecting adult Carukia barnesi from double Island using underwater lights at night, return them to the lab and induce spawning in them. Once spawned, the venom from these adult animals will be collected from both the bell and tentacles. Polyps will be produced and induced to asexually divide to produce high quantities of polyps. A subsample of these polyps will then be exposed to an indole (from my passed published work 9 to induce metamorphosis and the production of the medusa. Nematocysts will then be extracted from the polyps and medusa and along with the nematocysts extracted from the filed collected adults, venom profiles will be produced for these stages using SDS page gel, HPLC and mass spectroscopy. This will then allow me to compare the venom profiles these life stages, increasing the knowledge around why variations in the syndrome may exist (i.e. juvenile C. barnesi may have different venom). Similarly, the results from these experiments may well cast light on what the prey items may be for juvenile medusa as comparison of their venom profile to that of polyps will allow us to determine if the food for polyps is similar to that of juvenile medusa.
Lead researcher: Melissa Piontek (PhD Candidate)
Currently there is little research on human models challenged with C. fleckeri venom. This research aims to contribute knowledge to the underlying mechanisms of action that may be contributing to the human systemic and fatal pathology from C. fleckeri envenomation. Cellular models of the human nervous system and immune system will be employed to reveal how the venom effects cells that are present at the site of a sting and how they may lead to the cardiovascular symptoms.
Cells will be treated with whole venom, and subsets of different venom components, then analyzed using RNA sequencing which can quantify thousands of changes that occur within the cells.
Outcomes from this research may reveal mechanisms which can be targeted with new or existing treatments for C. fleckeri envenomation. One of the two molecules that we have isolated from C. fleckeri was reported in a study examining nine other species of box jellyfish therefore, treatment targeted for the mechanism may also be applicable to envenomation by these other species. Furthermore, this research may also contribute to understanding of pain pathways as venom toxins stimulate pain pathways; particularly with the discovery of the CGAs in C. fleckeri venom which are similar to glutamate- a key neurotransmitter in pain sensitization.
2021
Lead researcher: Emily O’Hara (PhD Candidate)
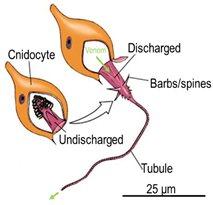
Jellyfish have stinging organelles called the nematocysts which have spines and a tubule which can penetrate prey to inject venom. To fully understand the ecological role of these organelles and further comprehend the role venom injection plays in sting victims, this project aims to image and 3d print both the discharged and undischarged nematocysts from both polyp and medusa life stages of the irukandji jellyfish Carukia barnesi and Malo maxima.
Hypotheses exist that suggest that a key aspect of the Irukandji sting, an approximately 20 minute delay between sting and symptom onset, is not a phenomenon rooted in the venom, but in the venom delivery system itself. The notion being that in the big box jellyfish Chironex fleckeri for example, the venom is ejected out of the sides of the discharged nematocyst tubule, injecting directly into a victim’s blood stream, thus the symptom onset is immediate.
In contrast, the nematocysts from Irukandji species are theorised only to eject venom directly out of the tip of the discharged tubule, injecting into the muscle which then takes time to work its way into the circulatory system, thus the delay in symptoms. However, there is currently only anecdotal evidence to support this.
By producing a 3d realisation of the nematocyst, we can determine the true venom delivery mechanism, providing significant advancement in the knowledge and understanding of not only the venom ecology of this animal, but its direct action in human envenomations.

Lead researcher: Scott Morrissey (PhD Candidate)
Cubozoan jellyfish are elusive by nature and as a result many challenges exist with respect to detection, which is of concern considering the threat they pose to humans. The polyps of the deadly Chironex fleckeri represent the longest-lived stage of jellyfish and are the source of medusa (adult form of Chironex fleckeri). However, there is almost no information available on the benthic polyp stage of this species.
This project will apply the innovative genetic technique, environmental DNA (eDNA) sampling to detect Chironex fleckeri medusae and polyps in waters with high tourist visitation, this being Magnetic Island off the coast of Townsville, Queensland. Should this technique prove successful it will remove the need to physically observe jellyfish in a location and will allow for quicker and increased detection. The study will also utilise the eDNA technique to examine the spatial patterns of jellyfish occurrence, and ground truth the accuracy of eDNA to determine the abundance of medusae within Horseshoe Bay.
Further, oceanographic modelling, that incorporates the decay of eDNA, will provide greater knowledge of ‘DNA halos’ that signals proximity to targeted polyp beds and jellyfish aggregations.
The specific aims of this project are as follows;
- Determine the source of polyps by sampling eDNA at a location with high tourist visitation.
- Determine spatial patterns of jellyfish occurrence, and potentially abundance, within and near a popular area for swimming, Horseshoe Bay, Magnetic Island.
- Ground truth the accuracy of eDNA to detect and determine the abundance of medusae.
- Develop oceanographic modelling of eDNA dispersal from source areas within the study area.
You can follow Scott's adventures on Twitter @ScottJMorrissey
2020
Lead researcher: Scott Morrissey (PhD Candidate)
Cubozoan jellyfish are elusive by nature and as a result many challenges exist with respect to detection, which is of concern considering the threat they pose to humans. The polyps of the deadly Chironex fleckeri represent the longest-lived stage of jellyfish and are the source of medusa (adult form of Chironex fleckeri). However, there is almost no information available on the benthic polyp stages of this species.
This project will apply the innovative genetic technique, environmental DNA (eDNA) sampling to detect Chironex fleckeri polys in the waters off Mapoon in far norther Queensland. Should this technique prove successful it will remove the need to physically observe jellyfish in a location and will allow for quicker and increased detection. The study also allows for investigation into the ecology of the polyp life history stage of this jellyfish which currently, is almost unknown.
Further, oceanographic modelling, that incorporates the decay of eDNA, will provide greater knowledge of ‘DNA’ halos’ that signals proximity to targeted polyp beds and jellyfish aggregations.
The specific aims of this project are as follows;
1. Determine the rate of decay of eDNA for Chironex fleckeri medusa.
2. Demonstrate experimentally in the laboratory and field that we can detect the presence/absence and relative abundance of Chironex fleckeri medusae and polyps using eDNA.
3. Undertake oceanographic modelling of eDNA halos incorporating eDNA decay to determine dispersal distances of eDNA from point-sources (e.g. beds of polyps).
You can follow Scott's adventures on Twitter @ScottJMorrissey

2019
Lead researcher: Olivia Rowley (PhD Candidate)
The Australian box jellyfish (Chironex fleckeri) is a large active jellyfish common to the calm inshore waters of Northern Australia. Considered the most venomous animal in the world, the box jellyfishes’ deadly reputation is due to its potent venom, which while highly adapted for capturing large active prey such as prawns and fish, is responsible for a number of serious stings annually.
Currently Surf Life Saving Queensland relies on beach goers swimming within nets, wearing full body stinger suits and regular beach drags to ensure swimmer safety. However, suits are often disregarded, drags are time intensive and, it is not feasible to net every beach. So how do we protect our beachgoers?
Unmanned aerial vehicles (UAV’s) are becoming commonplace as marine monitoring tools and have proven effective for the detection of a large number of marine animals. In this project we will be testing the viability of ‘off the shelf’, consumer grade, drones to detect box jellyfish and protect our swimmers.
You can follow Olivia on Twitter to see her progress: @oliviarowley4


Lead researcher: Emily O’Hara (PhD Candidate)
From the lethal big box jellyfish Chironex fleckeri to the tiny irukandji Carukia barnesi, jellyfish species around Northern Queensland pose a major health hazard to humans. These animals possess microscopic stinging organelles filled with venom, which is injected into their prey and often inadvertently humans. Venom is made up of multiple toxins and variation in this venom composition has been documented in a number of animals between genders, life stages, diet and geographic location, but environmental temperature has been scarcely considered, and never within jellyfish.
With global warming occurring at an unprecedented rate, it is important to understand if the venom of these dangerous animals may change as temperatures increase. Any changes in venom could alter the severity of these animals stings. This project will grow young jellyfish (polyps) through to adulthood at different temperatures, then use molecular techniques to analyse the venom at each temperature.

View more of the projects the Trust has supported.