Research and Innovation Services Partner with us Our Technologies Hyperstable Vaccines
Hyperstable Vaccines
- Future Students
- JCU Global Experience
- International Students
- Open Day
- How to apply
- Pathways to university
- Virtual Open Day
- Living on Campus
- Courses
- Publications
- Scholarships
- Parents and Partners
- JCU Heroes Programs
- Aboriginal and Torres Strait Islander in Marine Science
- Elite Athletes
- Defence
- Current Students
- New students
- JCU Orientation
- LearnJCU
- Placements
- CEE
- Unicare Centre and Unicampus Kids
- Graduation
- Off-Campus Students
- JCU Job Ready
- Safety and Wellbeing
- JCU Prizes
- Professional Experience Placement
- Employability Edge
- Art of Academic Writing
- Art of Academic Editing
- Careers and Employability
- Student Equity and Wellbeing
- Career Ready Plan
- Careers at JCU
- Partners and Community
- JCU-CSIRO Partnership
- Alumni
- About JCU
- Reputation and Experience
- Chancellery
- Governance
- Celebrating 50 Years
- Academy
- Indigenous Engagement
- Education Division
- Graduate Research School
- Research and Teaching
- Research Division
-
Research and Innovation Services
- About Research and Innovation Services
- Partner with us
- Innovate with us
- Research Grants, Tenders and Funding
- Ethics and Research Integrity
- Research Contracts
- Discover our Research and Testing Facilities
- JCU Ideas Lab
- Find an Expert
- Contact Research and Innovation Services
- FAQs and Fact Sheets
- GECO
- CASE
- College of Business, Law and Governance
- College of Healthcare Sciences
- College of Medicine and Dentistry
- College of Science and Engineering
- CPHMVS
- Anthropological Laboratory for Tropical Audiovisual Research (ALTAR)
- Anton Breinl Research Centre
- Agriculture Technology and Adoption Centre (AgTAC)
- Advanced Analytical Centre
- AMHHEC
- Aquaculture Solutions
- AusAsian Mental Health Research Group
- ARCSTA
- Area 61
- Lions Marine Research Trust
- Australian Tropical Herbarium
- Australian Quantum & Classical Transport Physics Group
- Boating and Diving
- Clinical Psychedelic Research Lab
- Centre for Tropical Biosecurity
- Centre for Tropical Bioinformatics and Molecular Biology
- CITBA
- CMT
- Centre for Disaster Solutions
- CSTFA
- Cyclone Testing Station
- The Centre for Disaster Studies
- Daintree Rainforest Observatory
- Fletcherview
- JCU Eduquarium
- JCU Turtle Health Research
- Language and Culture Research Centre
- MARF
- Orpheus
- TESS
- JCU Ideas Lab
- TARL
- eResearch
- Indigenous Education and Research Centre
- Estate
- Work Health and Safety
- Staff
- Discover Nature at JCU
- Cyber Security Hub
- Association of Australian University Secretaries
- Services and Resources Division
- Environmental Research Complex [ERC]
- Foundation for Australian Literary Studies
- Gender Equity Action and Research
- Give to JCU
- Indigenous Legal Needs Project
- Inherent Requirements
- IsoTropics Geochemistry Lab
- IT Services
- JCU Webinars
- JCU Events
- JCU Motorsports
- JCU Sport
- Library
- Mabo Decision: 30 years on
- Marine Geophysics Laboratory
- Office of the Vice Chancellor and President
- Outstanding Alumni
- Pharmacy Full Scope
- Planning for your future
- Policy
- PAHL
- Queensland Research Centre for Peripheral Vascular Disease
- Rapid Assessment Unit
- RDIM
- Researcher Development Portal
- Roderick Centre for Australian Literature and Creative Writing
- Contextual Science for Tropical Coastal Ecosystems
- State of the Tropics
- Strategic Procurement
- Student profiles
- SWIRLnet
- TREAD
- TropEco for Staff and Students
- TQ Maths Hub
- TUDLab
- VAVS Home
- WHOCC for Vector-borne & NTDs
- Media
- Copyright and Terms of Use
- Australian Institute of Tropical Health & Medicine
- Pay review
Technology platform for vaccine design useful for multiple indications in infectious diseases, cancer, & autoimmunity.
Proof of concept demonstrated with a potentially universal oral influenza vaccine candidate.
Background
Worldwide vaccination programs save millions of lives annually. However, temperature-sensitivity and need for delivery by injection for these sensitive biological compounds cause substantial hurdles in development and deployment. WHO estimates that up to 80% of cost of a vaccination campaign is from the cold chain & 50% of the wastage is from breaks in the cold chain.
Furthermore, some vaccines in market have significant limitations, e.g. influenza vaccines that require seasonal updates, making scale-up and stockpiling difficult.
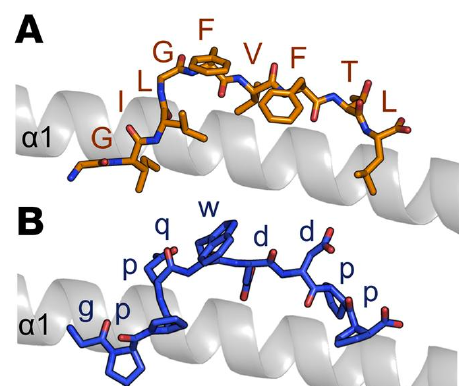
JCU researchers have used synthetic biology to generate hyper-stable vaccines that bypass the need for cold chain transport and offer the potential for oral delivery. The technology is a platform technology based on D-amino acid combinatorial chemistry. The vast majority of proteins in nature are constructed from L-amino acids, which are highly susceptible to degradation by endogenous proteases. In contrast, D-amino acids are intrinsically resistant to degradation. We are creating synthetic antigens using D-aminoacids that achieve similar structural configurations & offer more stable alternatives (Fig. 1).
Fig.1: Structural modelling indicates that the native (A) and our synthetic (B) agonist form similar overall confirmations despite differing primary sequences.
We have early-stage results with influenza & EBV. The summary of results with influenza vaccine candidate are as follows: We reverse engineered a conserved CD8+ T cell agonist into fully artificial antigen. The synthetic agonist stimulated and expanded an archetypal repertoire of polyfunctional human influenza virus-specific CD8+ T cells more than natural antigen. Mice vaccinated with artificial antigen survived a lethal influenza challenge significantly more than a control group. Moreover, the synthetic agonist was immunogenic after oral administration & stable in varying temperature conditions (room temperature & freezing).
The benefits of the technology is to create synthetic antigens that offer stable & immunogenic alternatives to conventional vaccines.
Advantages of our influenza vaccine candidate are:
- Hyperstable
- Potential oral activity
- Potential universal vaccine
- May not require frequent changes
- Easy to scale up/stock supplies against pandemics
This is a platform technology applicable to developing vaccines for infectious diseases & cancer, and other peptides. The pipeline includes candidates for EBV, CMV, cancer immunotherapy, etc.
We are seeking funding & support to take forward the lead candidate through preclinical development, as well as collaboration on pipeline development.
Publications
J Clin Invest. 2018;128(4):1569-1580. https://doi.org/10.1172/JCI91512
Patent
WO2018064718: Peptide Libraries and methods of use (Filed from QIMR)